Latest News
Research & Innovation
Global trial co-led by Massey researcher expected to set new standard of breast cancer care
Dec 5, 2018

A phase 3 international clinical trial co-led by a VCU Massey Cancer Center researcher is expected to change the way a specific type of breast cancer is treated in a subset of patients. The trial, known as KATHERINE, found that a drug called trastuzumab emtansine reduced the risk of cancer recurring by 50% in patients with HER2-positive early-stage breast cancer who had residual disease after receiving neoadjuvant therapy.
As global co-chair of the trial, Massey physician-scientist Charles Geyer, M.D., presented the results at the 2018 San Antonio Breast Cancer Symposium, held December 4-8. Data will be submitted to health authorities around the world, including the U.S. Food and Drug Administration and European Medicines Agency, and the study has been accepted for publication in The New England Journal of Medicine.
“I believe these results will be practice-changing,” said Geyer, a medical oncologist specialized in breast cancer and the associate director for clinical research at Massey. “The results should form the foundation of a new standard of care in patients with residual invasive breast cancer following neoadjuvant therapy.”
The trial tested the efficacy and safety of the antibody-drug conjugate trastuzumab emtansine (also known as T-DM1 and Kadcyla), which is a combination of the standard FDA-approved drug trastuzumab (also known as Herceptin) and a chemotherapy drug called DM1. T-DM1 is currently approved by the U.S. Food and Drug Administration to treat patients with metastatic HER2-positive breast cancer following previous treatment with trastuzumab and a taxane type of chemotherapy.
The study compared T-DM1 with trastuzumab as post-operative, or “adjuvant”, therapy for early stage HER2-positive breast cancer patients. The aim of the trial was to see if T-DM1 is better than trastuzumab at stopping cancer from coming back in patients with HER2-positive breast cancer who still had some cancer remaining after neoadjuvant therapy, which is treatment done before surgery to try to shrink a tumor to make it easier to remove.
One in six women with breast cancer – about 15 percent of all breast cancers – are diagnosed as HER2-positive. HER2-positive refers to patients whose cancer has large amounts of a protein called Human Epidermal Growth Factor Receptor 2 (HER2). The abnormal expression of the HER2 protein drives their tumor’s growth and makes the cancer cells more aggressive and less responsive to chemotherapy. Standard treatments for HER2-positive patients include chemotherapy and trastuzumab, more recently combined with pertuzumab. Trastuzumab is a specially designed antibody that binds to the abnormal amounts of the HER2 protein present in these tumors. Blocking this protein improves the activity of chemotherapy. After patients complete preoperative chemotherapy with trastuzumab, they undergo surgery to remove any remaining tumor in the breast or in the lymph nodes under the arm. If there is no residual cancer found in the breast or lymph nodes after surgery, patients typically do well with little chance for cancer recurrence. However, if cancer is still present, patients have an increased risk of recurrence.
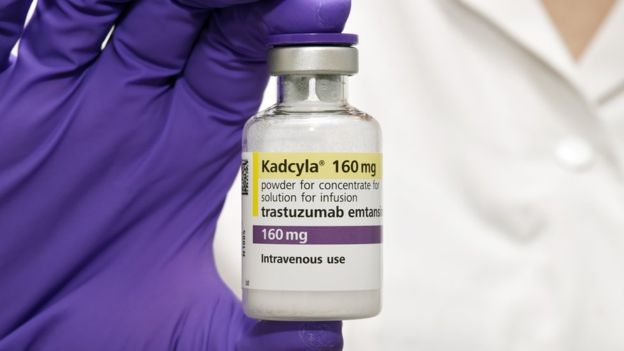
In KATHERINE, researchers tested whether treating patients at higher risk for recurrence following neoadjuvant therapy (based on the finding of persisting invasive breast cancer after surgery) with T-DM1 instead of the standard therapy of trastuzumab would reduce the risk of recurrence without an unacceptable increase in toxicities.
“The key to the success of T-DM1 is that the linker – the piece of the molecule that hooks the chemotherapy drug onto the antibody – is designed to come apart only when the molecule gets inside a cell. It doesn’t fall apart in your blood stream and harm healthy cells,” said Harry Bear, M.D., Ph.D., who was the local principal investigator for the trial at Massey. Bear is also the Dr. Walter Lawrence, Jr., Chair in Surgical Oncology and a member of the Developmental Therapeutics research program at Massey as well as professor of surgery and professor of microbiology and immunology at the VCU School of Medicine.
Results of the trial showed that substituting T-DM1 for adjuvant trastuzumab in HER2-positive patients significantly reduced the risk of invasive disease recurrence or death.
“We found that T-DM1 improved the three-year invasive disease-free survival (IDFS) rate by 11.3 percentage points (77 percent with trastuzumab and 88.3 percent with T-DM1),” explained Geyer, who is also the Harrigan, Haw, Luck Families Chair in Cancer Research at Massey, a member of Massey’s Developmental Therapeutics research program and professor in the Division of Hematology, Oncology and Palliative Care at the VCU School of Medicine.
The side effects in KATHERINE were similar to what occurs when T-DM1 is used for treating patients with metastatic disease. Side effects included drops in platelet count, sensory neuropathy and elevations in liver enzymes. Most of these toxicities are grade 1 or 2 and are resolved following reduction of the dose of T-DM1 or discontinuation.
The KATHERINE trial is an open-label study of 1,486 patients with residual HER2-positive breast cancer randomized to complete a year of adjuvant therapy with either trastuzumab (the current standard) or T-DM1. The study opened in April 2013 and will continue to collect patient data until April 2023.
KATHERINE was sponsored by F. Hoffmann La Roche/Genentech and was led by the National Surgical Adjuvant Breast and Bowel Project Foundation, Inc., and the German Breast Group. Geyer serves (uncompensated) on breast cancer advisory Boards for F. Hoffmann La Roche/Genentech and receives travel reimbursement.
Written by: Jenny Owen
Related News
Research & Innovation
Robert A. Winn Excellence in Clinical Trials Program Annual Convening highlights long-term, dynamic public-private partnershipNov 24, 2025
Research & Innovation
Commonwealth of Virginia Cancer Research Conference unites cancer researchers, students, and trainees from across the stateNov 21, 2025
Research & Innovation
Targeted drug could benefit pediatric and young adult patients with invasive soft tissue cancerNov 18, 2025

Get access to new, innovative care
Treatments in clinical trials may be more effective or have fewer side effects than the treatments that are currently available. With more than 200 studies for multiple types of cancers and cancer prevention, Massey supports a wide array of clinical trials.
Find a provider
Massey supports hundreds of top cancer specialists serving the needs of our patients. Massey’s medical team provides a wealth of expertise in cancer diagnosis, treatment, prevention and symptom management.
