Latest News
Clinical, Center News & Funding
Massey becomes first in Virginia to offer FDA-approved CAR T-cell therapy
Jul 2, 2018
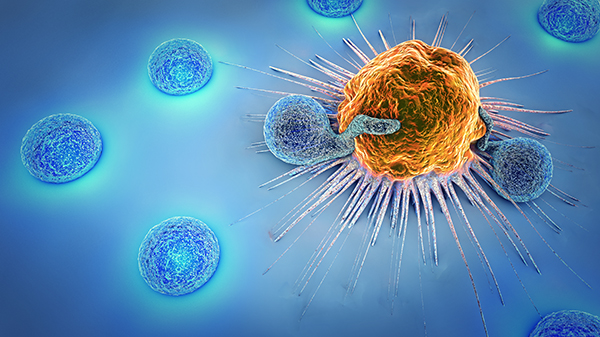
CAR T-cell therapy has proven very effective at treating advanced blood cancers in both children and adults. These types of cancers are usually treated successfully with chemotherapy, but in some cases conventional approaches do not work. That’s when CAR-T cell therapy can be a patient’s best option and may even allow many patients to avoid a bone marrow transplant.
A major advantage of CAR T-cell therapy is that it is a single infusion that usually requires one week of inpatient care, as opposed to the six months or more of chemotherapy, specialized post-transplant care and lifestyle restrictions typically required for patients with non-Hodgkin’s lymphoma and childhood leukemia patients no longer responding to conventional therapies.
Also, the benefits of CAR T-cell therapy may last for many years. Since the cells can persist in the body long term, the patient’s own immune system is effectively reprogrammed to recognize and attack cancer cells to prevent relapse.
McCarty adds that CAR T-cell therapies, while extremely promising, are viewed as another tool available to doctors in Massey’s Cellular Immunotherapies and Transplantation Program. Each patient is carefully evaluated before the team recommends the combination and sequence of treatments they believe will provide the best possible outcomes and quality of life. For some, CAR T-cell therapy may be the primary form of treatment while others may benefit more from stem cell transplantation or a combination of the two approaches.
“We extensively evaluate each patient and work with their referring physicians to create a personalized treatment plan that carefully weighs the risks and benefits of each approach,” says hematologist-oncologist Gary Simmons, D.O., deputy director of the program. “While CAR T-cell therapies can potentially offer lasting and durable remission from cancer, they also can carry a risk for serious side effects related to how they work.”

Effectively managing the potential side effects of CAR T-cell therapies requires collaboration among many different specialties, which is why these gene therapies are typically only found at NCI-designated cancer centers like Massey and large academic hospitals. The most notable and serious side effect is cytokine release syndrome (CRS), also known as a “cytokine storm,” which is an inflammatory response caused by the immune system. The symptoms may appear benign at first, but can rapidly worsen and lead to neurological complications. This process of cytokine hyperactivity is actually a necessary part of expanding the activity and longevity of the CAR T-cells, so having comprehensive team that can modulate the impact of this process while supporting the patient is a key component to the therapies’ success.
“Massey’s strong neuro-oncology team and long-standing clinical collaborations with our critical care team and pediatric experts at Children's Hospital of Richmond at VCU are key parts of our comprehensive program and make us an ideal site for this novel treatment,” says McCarty. “Patients receiving CAR T-cell therapy at Massey will stay at the hospital for one week after infusion, and they must remain within a 30-minute drive of Massey for an additional month in order for the care team to be able to rapidly and effectively address the unique side effects that can occur with this therapy. During that time, they are advised to visit us at the onset of even the mildest symptoms to receive immediate access to care.”
Due to the complexities of CAR T-cell therapies and stem cell transplantation, Massey’s cellular immunotherapy and transplant coordinators guide patients and their caregivers through the entire process. They help coordinate medical records, schedule tests, navigate insurance, assist with transportation, arrange treatment and follow-up appointments, manage side effects, develop survivorship plans and more. VCU Massey’s distinction as a center for complex and rare cancers allows many different therapeutic disciplines to contribute to the care of these patients and improve their experience and outcomes from this potent and novel therapy.
For more information about CAR T-cell therapies or other treatments available through Massey’s Cellular Immunotherapies and Transplantation Program, please visit www.MasseyCIT.org, call (804) 628-2079 or email judith.davis@vcuhealth.org.
Written by: John Wallace
Related News

Get access to new, innovative care
Treatments in clinical trials may be more effective or have fewer side effects than the treatments that are currently available. With more than 200 studies for multiple types of cancers and cancer prevention, Massey supports a wide array of clinical trials.
Find a provider
Massey supports hundreds of top cancer specialists serving the needs of our patients. Massey’s medical team provides a wealth of expertise in cancer diagnosis, treatment, prevention and symptom management.
